Why do people still doubt generic drugs?
It’s 2026. Nine out of every ten prescriptions filled in the U.S. are for generic drugs. Yet, nearly 80% of doctors say patients still worry these meds aren’t as good as the brand-name versions. That’s not a data problem. It’s a perception problem.
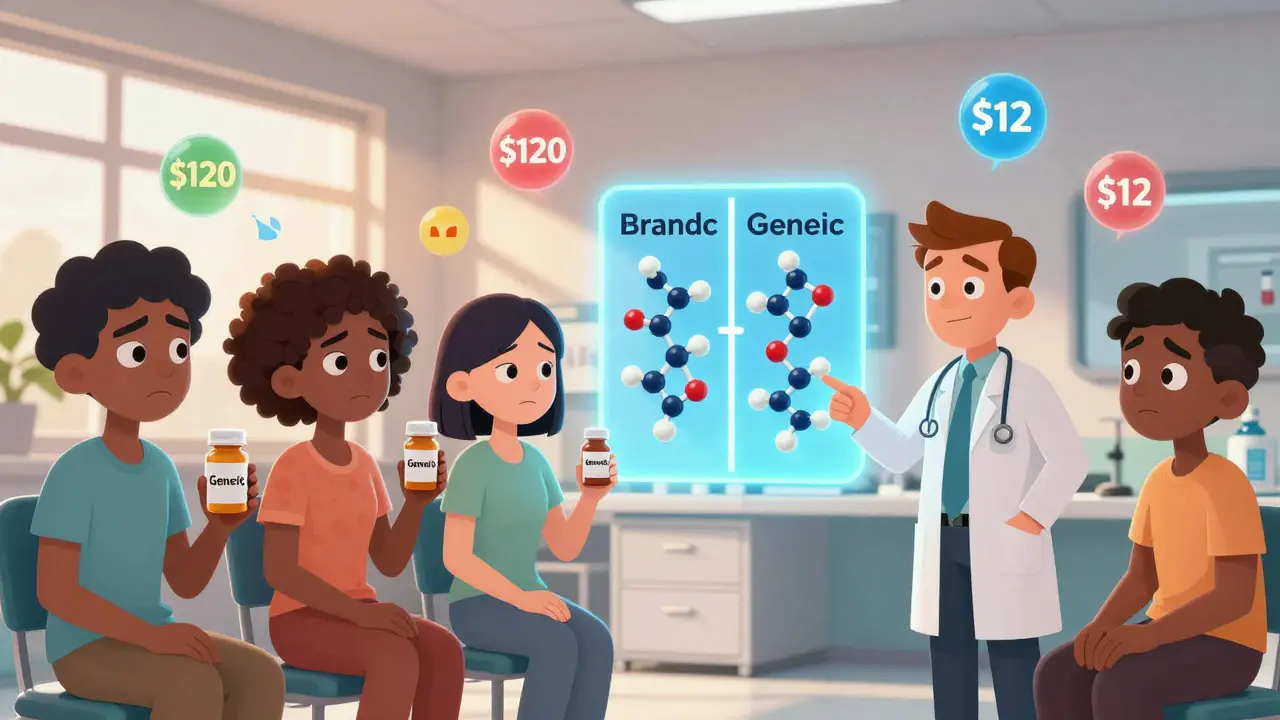
Generic drugs contain the exact same active ingredients as their brand-name cousins. They’re tested to match in strength, speed, and effect. The FDA requires them to be bioequivalent - meaning they work the same way in your body. But that doesn’t matter if you don’t believe it.
People remember the old days. Back in the 1990s, some generics looked different. Pills were cheaper, sometimes with odd shapes or colors. Some patients reported feeling “off” after switching. Those experiences stuck. Even though today’s generics are made in the same high-tech facilities as brand-name drugs - often by the same companies - the mental image hasn’t changed.
The real cost difference isn’t what you think
Generic drugs save patients and the system billions. In 2025, they made up 90% of prescriptions but only 12% of total drug spending. That means for every $100 spent on medicines, $88 goes to brand-name drugs - even though most pills taken are generics.
Why? Because the most expensive drugs aren’t the ones you take daily. They’re the specialty ones: injectables for cancer, biologics for autoimmune diseases, GLP-1 drugs for weight loss and diabetes. These can cost $10,000 a month. Generics for blood pressure or cholesterol? $5 to $15 a month.
But here’s the twist: the public hears more about the $10,000 drugs. Ads scream about them. News stories focus on their prices. Meanwhile, the quiet heroes - the generics that keep millions of people healthy without breaking the bank - go unnoticed.
Complex generics and biosimilars are changing the game
Today’s generics aren’t just pills anymore. In 2025, the FDA approved six new biosimilars for denosumab - the drug used for osteoporosis and bone cancer. Brands like Prolia and Xgeva had patents expire, and now companies like Bildyos, Aukelso, and Enoby are offering the same drug at 15-30% less.
Biosimilars aren’t simple copies. They’re made from living cells, like the original. Manufacturing them is incredibly complex. They require the same precision as making a Swiss watch. And yet, many patients still think, “If it’s not the brand, it’s not the same.”
Hospitals are leading the shift. In oncology units, nurses now routinely give generic injectables. Patients get the same treatment. Same outcomes. Same side effects. But the hospital saves enough to treat five more people. That’s not theory - it’s happening right now.

Why trust is broken - and how to fix it
People don’t distrust generics because they’re dumb. They distrust them because they’ve been sold a story.
Brand-name companies spent decades building trust through slick ads, doctor visits, and patient support programs. Generics? They didn’t advertise. They didn’t have mascots. They were the quiet option - the one your insurance pushes.
But that’s changing. A 2025 pilot by the American Medical Association trained doctors to explain generics to patients using simple, clear language: “This pill has the same active ingredient as the brand. It’s made to the same standards. The only difference? You’ll pay $12 instead of $120.” After the training, patient concerns dropped by 35%.
It’s not about shaming people for thinking generics are inferior. It’s about replacing myths with facts - in a way that feels personal.
The future: transparency, tech, and trust
Two big ideas are leading the next wave of change.
First: transparency in supply chains. Companies like CivicaScript are cutting out middlemen. They partner directly with hospitals to make generic drugs at cost. No profit margin. No markups. Just affordable, reliable meds. Their model has cut drug shortages by 40% in pilot regions. When patients see the same bottle in the hospital, with a label that says “Made by Civica,” trust grows.
Second: blockchain and AI. Imagine scanning a pill bottle and seeing its full journey: where it was made, when it was tested, who inspected it, and how many batches passed FDA checks. That’s not sci-fi - it’s being tested now. Digital tracking doesn’t just prevent counterfeits. It builds confidence.
And then there’s domestic production. Over 80% of generic drug ingredients used to come from India and China. That’s changed. New U.S.-based manufacturing plants are opening, especially for injectables. More local production means fewer shortages. Fewer shortages mean fewer missed doses. Fewer missed doses mean better health. And better health builds trust.

What’s next? It’s not about price anymore
Generic drug prices have dropped so far that they’re hitting a floor. Experts say they’ll stabilize. That means the next battle isn’t about cost - it’s about value.
Patients don’t care about a $10 difference if they think the drug won’t work. But they’ll pay more - or stick with a brand - if they feel safe and understood.
The future belongs to companies and clinics that treat perception like a medical condition. You don’t just hand out a pill. You explain it. You show it. You track it. You make it visible.
When a patient sees their generic insulin bottle and knows it’s made in Ohio, tested in a lab with the same standards as the brand, and delivered without delay - they stop worrying. They start healing.
It’s time to stop calling them “generic”
That word - “generic” - is the problem. It sounds like “basic,” “plain,” “low quality.”
What if we started calling them “equivalent” or “standardized” or “value-matched”? What if the FDA started using “bioequivalent medication” on labels? Language shapes belief.
When you hear “brand-name,” you think “trusted.” When you hear “generic,” you think “cheap.” But what if you heard “FDA-approved equivalent”? You’d think “reliable.”
Changing the name isn’t just marketing. It’s medicine.






I've seen what happens when you switch to generics. My uncle went from brand-name statin to the generic. Two weeks later, he was in the ER with muscle pain so bad he couldn't lift his arms. They said it was 'coincidental.' Yeah, right. Same active ingredient? Sure. But the fillers? The binders? Who tests those? I'm not trusting my health to a mystery pill.
The narrative here is charmingly naive. You speak of bioequivalence as if it were a mathematical certainty. Yet, in pharmacokinetics, even a 5% deviation in absorption can trigger clinical consequences in narrow-therapeutic-index drugs. The FDA’s 80-125% bioequivalence window is not a guarantee of therapeutic parity-it is a statistical compromise. And let us not forget: the same companies that manufacture generics often hold the patents on the branded versions. This is not a revolution. It is a rebranding of oligopoly.
I work in a rural clinic. We switched 80% of our prescriptions to generics last year. The patients? They were scared at first. One woman cried because she thought her blood pressure med was 'fake.' So we started showing them the FDA sticker on the bottle. We printed out the bioequivalence studies in plain language. We let them hold the brand and generic side by side. Within three months, not one patient asked to switch back. Trust isn't built with ads. It's built with patience and proof.
Let’s be real. The reason generics are trusted less is because America’s healthcare system is built on fear and profit. Brand-name drugs? They’re marketed like luxury cars. Generics? They’re the economy car that somehow still gets you to work. But if you’re not from a place where $120 vs $12 means the difference between eating or not? You don’t get it. We need to stop pretending this is about science. It’s about class. And until we fix that, no amount of blockchain will help.
I’ve been on generics for 12 years. Never had an issue. My thyroid meds, my BP, my antidepressants-all generic. I don’t need a lecture. I just need to know they’re safe. They are.
The FDA’s bioequivalence standards? They’re based on healthy volunteers in controlled trials. But what about diabetics? Elderly patients with renal impairment? Those with gut absorption issues? The data doesn’t reflect real-world polypharmacy. And don’t even get me started on how some generics are manufactured in the same facility as the brand-just on a different shift. It’s not a conspiracy. It’s just… sloppy science dressed up as policy. Also, 'CivicaScript'? Sounds like a startup that got a grant and a PR team. I’ll believe it when I see it on my prescription.
Simple truth: generics work. I’m a pharmacist in Mumbai. We use generics daily. Same results. Same safety. The problem is not the medicine. It’s the story. People think if it’s cheap, it’s weak. But a $5 pill that stops a heart attack? That’s not cheap. That’s genius.
Okay, so I’ve been reading this whole thing and I’m just sitting here thinking-how did we get so good at marketing pills that we forgot they’re supposed to be medicine, not status symbols? I had a friend who refused to take her generic antidepressant because it was a different color. Like, the color mattered more than whether she felt less anxious. And then we started showing her the FDA paperwork, the batch numbers, the fact that her pharmacy’s generic was made in the same building as the brand. She cried. Not because she was mad. Because she realized she’d been scared for years over nothing. It’s not about the pill. It’s about the fear we let companies plant in us. And honestly? I’m tired of it. We need to stop letting marketing define our health. We need to start trusting science. And maybe, just maybe, we need to stop calling them 'generic.' 'Standardized' sounds way less scary.