IPF Treatment Comparison Tool
Esbriet (Pirfenidone)
Primary Mechanism: Anti-inflammatory and anti-fibrotic; inhibits TGF-β-stimulated collagen production.
Dosage: 801 mg/day (267 mg three times daily) after 2-week titration.
Side Effects: Photosensitivity Rash Nausea Loss of Appetite Liver Enzyme Elevation
Effectiveness: ~30% slower FVC decline vs placebo.
Annual Cost: $110,000 - $130,000
Nintedanib (Ofev)
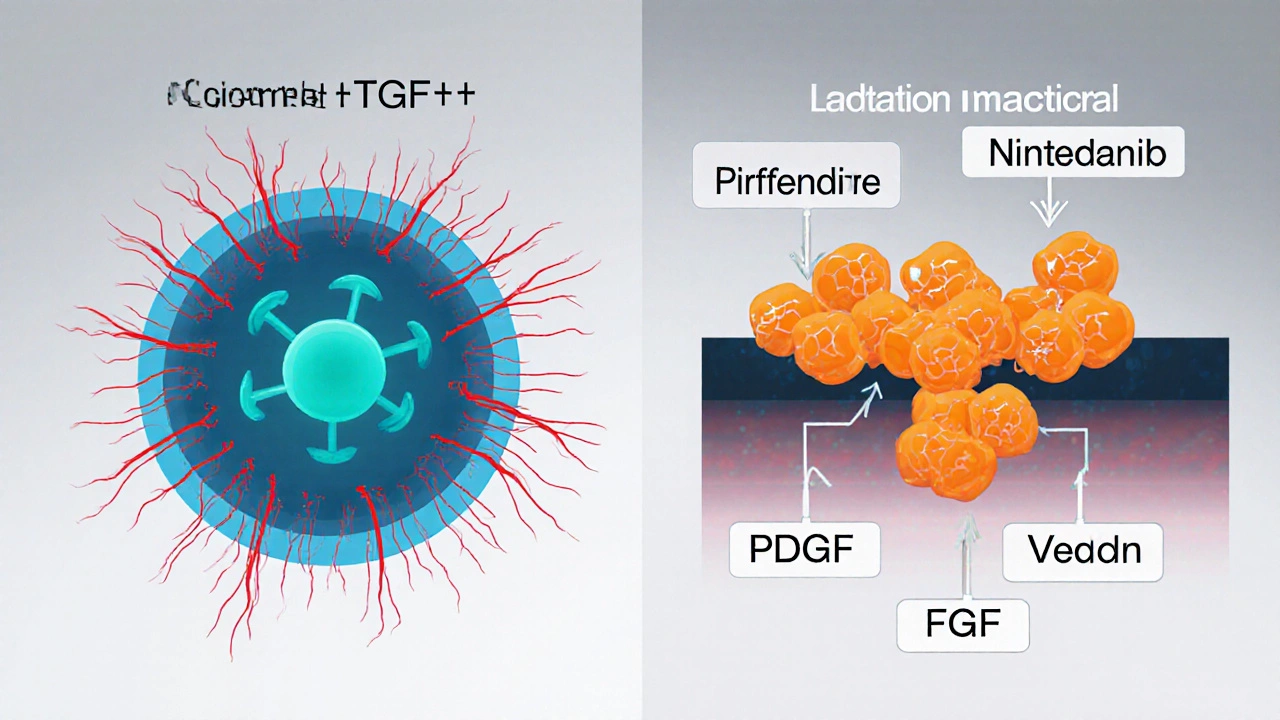
Primary Mechanism: Broad tyrosine-kinase inhibition; blocks PDGF, VEGF, FGF receptors.
Dosage: 150 mg twice daily with food.
Side Effects: Diarrhea Nausea Abdominal Pain Liver Enzyme Elevation
Effectiveness: ~40% slower FVC decline vs placebo.
Annual Cost: $95,000 - $115,000
Decision Factors
Recommended Treatment
Esbriet has become a go‑to option for people battling idiopathic pulmonary fibrosis (IPF), but it isn’t the only game‑changer on the shelf. This guide breaks down how Esbriet stacks up against its main rivals, what side‑effects to expect, and which drug might suit a particular health profile.
Quick Take
- Esbriet (pirfenidone) slows lung function decline by about 30% in most trials.
- Nintedanib (Ofev) offers a similar 30‑40% slowdown but comes with more gastrointestinal complaints.
- Both drugs cost roughly $100,000‑$130,000 per year in the U.S.; insurance coverage varies widely.
- Choosing the right drug hinges on liver health, stomach tolerance, and lifestyle factors like sun exposure.
- Combination therapy is still experimental; most clinicians stick to one agent at a time.
What is Esbriet (Pirfenidone)?
When the name Esbriet pops up, it refers to a medication whose active ingredient is pirfenidone, a synthetic small‑molecule that reduces lung scarring by dampening fibroblast activity and inflammation. Approved by the FDA in 2014 for IPF, Esbriet is taken in three daily doses after a titration period. Clinical trials-CAPACITY (2011) and ASCEND (2014)-showed a roughly 30% reduction in the rate of forced vital capacity (FVC) decline compared with placebo.
Key Alternatives on the Market
The only other drug with a full FDA indication for IPF is Nintedanib, sold under the brand name Ofev. Nintedanib is a tyrosine‑kinase inhibitor that blocks multiple growth factor receptors involved in fibrosis. It hit the U.S. market a year earlier, in 2015, after proving its own efficacy in the INPULSIS trials.
Other agents sometimes considered off‑label or in clinical trials include:
- Glucocorticoids (high‑dose steroids) - mainly for acute exacerbations.
- Antifibrotic combinations (e.g., pirfenidone + nintedanib) - still under investigation.
- Lung transplantation - the ultimate rescue for end‑stage disease.
How the Drugs Work: Mechanism at a Glance
| Attribute | Esbriet (Pirfenidone) | Nintedanib (Ofev) |
|---|---|---|
| Primary Mechanism | Anti‑inflammatory and anti‑fibrotic; inhibits TGF‑β‑stimulated collagen production. | Broad tyrosine‑kinase inhibition; blocks PDGF, VEGF, FGF receptors. |
| Typical Dose | 801mg per day (267mg three times daily) after 2‑week titration. | 150mg twice daily with food. |
| Key Side‑Effects | Photosensitivity, rash, nausea, loss of appetite, liver enzyme elevation. | Diarrhea, nausea, abdominal pain, liver enzyme elevation. |
| FVC Benefit (average) | ≈30% slower decline vs placebo. | ≈40% slower decline vs placebo. |
| FDA Approval Year | 2014 | 2015 |
| Annual U.S. Cost (2025 estimate) | $110,000 - $130,000 | $95,000 - $115,000 |
Side‑Effect Profiles: What to Expect
Both drugs share liver‑enzyme monitoring as a safety requirement, but their symptom footprints differ enough to tip the scales for many patients.
Esbriet tends to cause photosensitivity-patients report sunburn‑like rashes after brief exposure. Wearing sunscreen (SPF30+) and protective clothing is mandatory during the first few months. Nausea and loss of appetite also appear early, but they often settle once the body adjusts.
Nintedanib’s most common complaint is persistent diarrhea, which can lead to dehydration if not managed. Dose reduction or adding antidiarrheal agents (e.g., loperamide) usually helps. Some users notice a metallic taste.
Both agents can raise transaminases (ALT/AST). Routine labs every 4-6weeks for the first three months, then quarterly, are standard practice. If levels exceed three times the upper limit of normal, a pause or dose cut‑back is advised.
Effectiveness in Real‑World Practice
Real‑world registries, such as the IPF‑Registry, show adherence rates of about 70% for Esbriet and 65% for Nintedanib after one year. Reasons for discontinuation line up with side‑effect burden: photosensitivity and nausea for Esbriet; severe diarrhea for Nintedanib.
When it comes to hard outcomes-hospitalization, transplant-free survival-differences are modest. A 2023 meta‑analysis of 10cohort studies found a pooled hazard ratio of 0.86 for mortality with either drug versus placebo, indicating both are life‑prolonging but not dramatically distinct.
Cost, Insurance, and Access Considerations
Insurance formularies vary widely. Medicare PartD covers both drugs, yet copay structures differ: Esbriet often falls under a higher tier, resulting in out‑of‑pocket costs of $1,500‑$3,000 per month for patients without supplemental plans. Nintedanib may be placed on a lower tier, but manufacturers frequently offer patient‑assistance programs that can shave off $2,000-$4,000 annually.
For uninsured or underinsured patients, specialty pharmacies sometimes negotiate discount cards. Additionally, some state Medicaid programs list one drug as preferred, making the alternative significantly more expensive.
Choosing the Right Drug: Decision Checklist
- Liver health: If baseline ALT/AST are already high, the clinician might favor the drug with a slightly milder hepatic profile (generally Nintedanib).
- Gastro‑intestinal tolerance: Patients with chronic IBS or ulcerative colitis may lean toward Esbriet to avoid worsening diarrhea.
- Sun exposure: Outdoor workers or those living in sunny climates often opt for Nintedanib to sidestep photosensitivity.
- Cost & insurance: Review formulary tiering; a lower copay can outweigh marginal efficacy differences.
- Personal preference: Some patients dislike taking three pills a day (Esbriet) versus twice‑daily capsules (Nintedanib).
Future Directions: What’s on the Horizon?
New antifibrotic molecules-like pamrevlumab (a monoclonal antibody against CTGF) and PLN‐74809 (an integrin inhibitor)-are in PhaseIII trials. Early data suggest additive benefits when combined with either Esbriet or Nintedanib, but safety signals are still under review. For now, the current standard remains a single‑agent approach.

Frequently Asked Questions
How long does it take to see a benefit from Esbriet?
Patients usually notice a stabilization of lung‑function decline within 3-6months, but the full effect may take up to a year. Regular spirometry helps track progress.
Can I switch from Nintedanib to Esbriet (or vice‑versa) safely?
A wash‑out period of about two weeks is recommended to avoid overlapping toxicities. The transition should be supervised by a pulmonologist who will re‑check liver enzymes and renal function.
Is it okay to take both drugs together?
Current guidelines advise against routine combination because safety data are limited. Some clinical‑trial participants receive both, but that’s under strict monitoring.
What monitoring is required while on these medications?
Baseline liver function tests, then repeat every 4-6weeks for the first three months, followed by quarterly checks. Pulmonary function tests (FVC) are done every 3-6months to gauge efficacy.
Are there lifestyle changes that can boost the drug’s effect?
Avoid smoking, maintain a healthy weight, and engage in low‑impact aerobic exercise (e.g., walking, stationary cycling). Staying up‑to‑date with vaccinations (flu, pneumococcal) also reduces infection‑related lung decline.
Bottom line: both Esbriet and Nintedanib deliver meaningful slowing of IPF progression. The “best” choice hinges on individual tolerance, liver health, sun exposure, and insurance landscape. Talk with your pulmonology team, weigh the pros and cons listed here, and settle on the therapy that aligns with your daily life.






Ah, the nuanced dance between Esbriet and Nintedanib is nothing short of a pharmacological opera, worthy of a discerning connoisseur's attention. While the author dutifully outlines the mechanisms, one must also consider the subtle art of patient adherence, especially given Esbriet’s thrice‑daily regimen. The hepatic safety profile, albeit comparable, tips the scale marginally toward Nintedanib for those with pre‑existing ALT elevations. Moreover, the phototoxicity associated with pirfenidone is a non‑trivial occupational hazard for sun‑loving individuals. Ultimately, the decision rests upon a mosaic of clinical, lifestyle, and economic factors, each demanding a bespoke appraisal.
omg i feel ur pain, this is sooo overwhelming for anyone.
Let’s cut through the fluff: both drugs shave off a similar fraction of FVC decline, yet the side‑effect burden diverges dramatically. Esbriet’s photosensitivity can ruin a weekend beach plan, while Nintedanib’s diarrhea may keep you glued to the bathroom. From a cost perspective, neither is cheap, but formularies will dictate your out‑of‑pocket nightmare. If your liver enzymes are already dancing, steer clear of the one with a harsher hepatic imprint. In short, align the medication with your daily reality, not just the trial data.
One might reflect that the therapeutic choice mirrors a dialectic between bodily stewardship and existential pragmatism. The patient’s lived experience, replete with occupational sun exposure or gastrointestinal fragility, serves as the thesis; the pharmacologic agent operates as the antithesis. Synthesis emerges when clinicians integrate these dimensions into a shared decision‑making tableau. In this light, the comparative table is but a scaffold for a deeper, more human conversation.
PATRIOTIC, PRAGMATIC, POTENT!;
Totally get the vibe-if you’re juggling a 9‑to‑5 and want to keep things chill, the twice‑daily capsule might just be the smoother ride. Sun‑sensitive folks can breathe easy with Nintedanib, and you won’t have to panic about sudden rashes during your commute.
The analysis feels like a lukewarm drizzle on a desert of data; nothing spectacular, just the usual bland recitation of side‑effects and costs. I’d expect a sharper critique or at least an anecdotal sprinkle to spice things up.
Hey, I hear you. While the data swing between the two meds, it’s crucial to remember each patient’s narrative-some battle chronic IBS, others dodge sun like it’s a vampire. If liver labs are flickering, lean toward the drug with a milder hepatic footprint and keep the dialogue open with your pulmonologist.
Choosing between Esbriet and Nintedanib is akin to selecting a partner for a long journey through a treacherous landscape; you must consider not only the destination but also the daily terrain. First, acknowledge that both agents have demonstrated roughly a 30–40% deceleration in forced vital capacity decline, which, while statistically significant, remains a modest alteration in the natural history of IPF. Second, examine the dosing schedules: pirpirfenidone demands three divided doses, a regiment that can feel intrusive for those with disrupted routines, whereas nintedanib’s twice‑daily capsule aligns more comfortably with conventional medication habits. Third, weigh the side‑effect spectrums-photosensitivity from Esbriet can be mitigated with diligent sunscreen use, yet it still imposes a behavioral burden, especially for outdoor workers. Fourth, the gastrointestinal turmoil associated with nintedanib, notably persistent diarrhea, may necessitate adjunctive antidiarrheal therapy and vigilant hydration. Fifth, hepatic safety demands periodic monitoring for both drugs, but the clinical community often perceives nintedanib as possessing a slightly gentler liver profile, a nuance that could sway patients with pre‑existing enzyme elevations. Sixth, the financial calculus cannot be ignored; while the annual cost brackets overlap, insurance formularies may tip the scales dramatically based on tier placement and patient assistance programs. Seventh, consider the psychosocial dimension-patients who relish autonomy may prefer the ability to adjust dosing times, whereas those seeking simplicity might gravitate toward the bi‑daily capsule. Eighth, emerging real‑world registries suggest adherence rates hovering around 70%, with discontinuations frequently tied to adverse events, underscoring the importance of proactive side‑effect management. Ninth, the future horizon holds promise with novel antifibrotic agents and potential combination therapies, but until robust safety data emerge, monotherapy remains the standard. Tenth, shared decision‑making is the cornerstone; involve caregivers, pharmacists, and, crucially, the patient’s own values and lifestyle preferences. Eleventh, remember that lung transplantation remains an option for end‑stage disease, and optimizing medical therapy now can preserve candidacy for that ultimate rescue. Twelfth, regular pulmonary function tests every three to six months provide objective feedback on therapeutic efficacy. Thirteenth, vaccinations against influenza and pneumococcus are essential adjuncts, reducing infection‑driven exacerbations that can confound treatment outcomes. Fourteenth, maintain open communication channels with your healthcare team to promptly address any emerging concerns. Lastly, the “best” choice is not a universal truth but a personalized alignment of efficacy, tolerability, cost, and quality‑of‑life considerations.
Wow, that was a marathon of insight-honestly, I’m just trying to keep my coffee intake steady while navigating these meds. Your breakdown makes the complexity feel a bit more manageable, thanks.
Hey there, I totally get the overwhelm-balancing side‑effects with life’s daily grind is a real juggle. If diarrhea is your nightmare, make sure you have loperamide on standby and stay hydrated. On the flip side, if the sun is your nemesis, slather on SPF 30+ and consider a wide‑brim hat; the rash from pirfenidone can be a real mood‑killer. Whatever you choose, keep the dialogue open with your doc and don’t forget to celebrate the small wins.
Ah, the drama of medication choice strikes again-truly a theatrical production where the curtain never really falls. Still, a dash of humor helps us survive the playwright’s endless revisions.
Sure, because picking a drug is just as easy as choosing a flavor of ice cream.