Author: Taro White - Page 2

Switching to generic drugs can cause real side effects for some people, especially with high-risk medications like thyroid drugs, antiepileptics, and anticoagulants. Learn when to worry and what to do.

Learn the key differences between ischemic and hemorrhagic strokes, their causes, symptoms, treatments, and proven prevention strategies to reduce your risk. Time matters - act now.

Jan, 17 2026
An ANDA, or Abbreviated New Drug Application, is the FDA pathway that allows generic drugs to enter the market without repeating costly clinical trials. It saves billions annually and makes essential medicines affordable.

Learn how to choose between acetaminophen and NSAIDs for OTC pain relief. Discover which works best for headaches, arthritis, and inflammation-and how to avoid dangerous side effects.

Medications get pulled from the market when they're unsafe or ineffective. Learn how the FDA decides to withdraw drugs, why it took so long in the past, and how new 2023 rules are changing the game for patient safety.
Keep a medication journal to track how your body reacts when switching to generic drugs. Learn what to record, which medications need it most, and how to use your journal to talk to your doctor.

Jan, 11 2026
Many patients take separate generic pills instead of fixed-dose combinations (FDCs) to save money or get flexible dosing. But this 'de facto combination' can be risky - with untested interactions, lower adherence, and hidden safety issues. Here’s what you need to know.

Second and third generic drug manufacturers drive prescription prices down by 50% or more. Learn how competition after patent expiry saves billions and why fewer competitors can lead to higher costs.
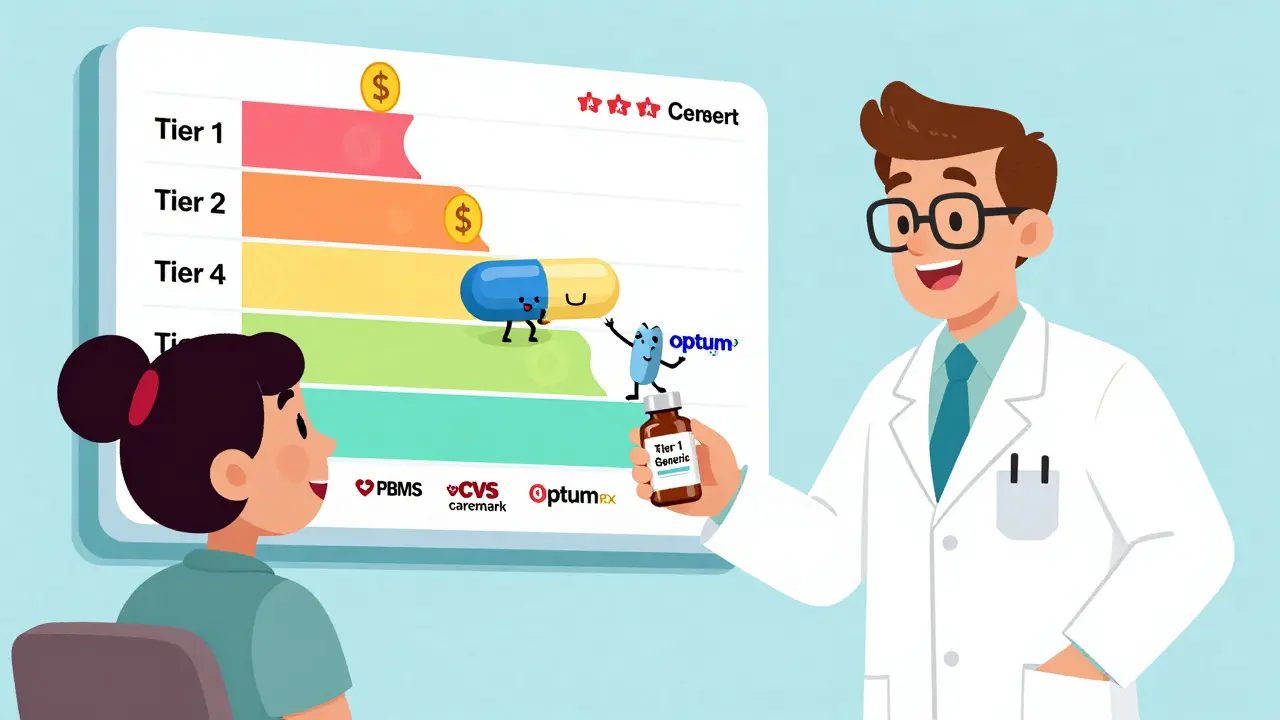
Insurers prefer generic drugs because they're 80-85% cheaper than brand names and just as effective. Learn how formularies work, why some drugs get blocked, and what you can do to save money.

Vertigo and dizziness are not the same. Vertigo means you feel spinning, often from inner ear issues like BPPV. Dizziness is lightheadedness, often from low blood pressure or anxiety. Knowing the difference can save you months of misdiagnosis.
